Mass and density of air
An “empty” vessel is filled - with air. When an open bottle is submerged in water, bubbles can be seen and heard leaving the bottles. Air is not "nothing".
In the following experiment, you should determine how heavy air is. With knowledge of the mass of a certain volume of air, you can calculate the mass of air in your classroom.
Experiment
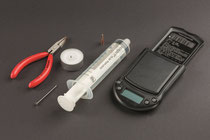
1. Fill the syringe with air by pulling the plunger to exactly 50 mL.
(more see below)
2. Heat the nail with the pliers over the tea light and melt a hole into the syringe plunger in line with the top of the syringe barrel. This is to make sure that it does not slip back.
3. Remove the nail again, push the plunger back and lock the syringe with a sealed connector.
4. Pull the syringe plunger to the 50 mL mark in the sealed syringe and fix it in that position by inserting the nail.
5. Weigh the empty syringe and record its mass.
6. Remove the syringe closure, weigh the syringe together with the closure and the nail. Record the mass.
First publication: 27.10.2009 Last modification: 19.07.2014
 Microscale Family 2013
Microscale Family 2013
