Air pressure and boiling temperature
Under standard conditions water
boils at about 100°C. In the mountains the boiling point is lower. On Mount Zugspitze (height: 2.962m ) water would boil
at 90°C, on Mount Everest at 70°C. At the Dead Sea, however, the boiling point exceeds 100°C. What are the reasons for these different values? To answer this question, you should perform the
following experiment.
Experiment
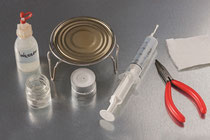
1. 5 mL of water are
heated to boiling in an ampoule placed on a stand above a candle burner. This stand is the wire of a sparkling wine
closure.
(more see below)
2. After extinguishing the flame, the ampoule is stoppered and connected with a 60-mL syringe.
3. By pulling the piston of the syringe, the boiling process can be restarted inside the ampoule for a long time.
First published: 2004 Last modification: 27.05.2015
 Microscale Family 2013
Microscale Family 2013
