Air can be compressed: Cartesian diver
Divers take with them on every dive a large volume of compressed air. Apart from enabling them to breathe they need it to regulate their inflatable vest: Depending on whether they wish to sink, to rise or to float they release or add some air.
In the following experiment, you construct a small `diver` and look at its behaviour when varying the external conditions.
Experiment
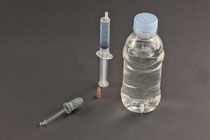
1. Fill the large syringe with exactly 5 mL of air.
2. Close it airtight with a sealed connector. (more see below)
3. Press the syringe plunger as far as it will go and note the volume of air in the syringe.Release the syringe plunger and note the volume again.
4. Repeat step 3 with a syringe that you have filled with 5 mL of water.
5. Fill the plastic bottle with water to about 3 cm below the neck.
6. Draw water to a height of about 0.5-1 cm by squeezing the rubber teat, into the glass pipette.
7. Put this ‘diver’ with the teat on the top into the bottle.
8. Close the bottle tightly and squeeze it as firmly as possible. What do you see?
9. Let go of the bottle so that it returns to its original shape.
10. Vary the pressure ratios again by alternately gently squeezing and letting go. What do you see?
First publication: 2007 Last modification: 18.07.2014
 Microscale Family 2013
Microscale Family 2013
