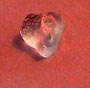
Gela´s first famous experiment
Super absorbent polymers (SAP) are polyacrylic acids with a low degree of cross-linking, - which are partially neutralized by NaOH. By adding water, 1 g of these macromolecular substances can absorb more than 200 g of water or aqueous solutions without dissolving. A hydrogel is formed, which does not release the liquid even under external pressure. The polymer molecules are cross-linked by covalent bondings, which are necessary to obtain their extremely low solubility. Due to the very low degree of cross-linking the structure remains flexible and can expand and compress.
1 grain of SAP..... ...1 grain of SAP.. ...1 grain of SAP .... 1 grain of SAP
.... untreatedt...................+ water..................+ 1M
NaOH................+ 1M HCl...
OBSERVATIONS and EXPLANATIONS
If water is replaced by solutions of different pH values, acid is the least absorbed. The reason for this is that there is an acid base reaction between the negative carboxylate groups of the SAP and the acid, resulting in neutral carboxyl groups. The electrostatic repellent forces in the SAP network are reduced, resulting in a lower ability to absorb water. At a pH of 8, SAP can absorb the highest amount of water.
 Microscale Family 2013
Microscale Family 2013