Kazuko Ogino
Microscale Experiments on Ion Exchangers
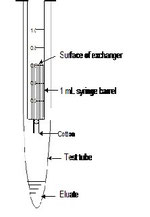
Construction of the column
1 mL syringes can serve as the column. The syringe is placed on a small test tube. No stand is necessary to hold the columns. Small piece of cotton is placed in the neck of the syringe. The SP- or QAE-Sephadex ion exchanger suspended in deionized water is placed in the syringe. Students can handle several columns at a time.
Experiments
Step 1: 3 drops of the CuSO4 solution are passed through QAE-column in Cl form. By the addition of deionized water to the column, blue Solution A is obtained. In this procedure sulfate anions (with minus-two-charge) are adsorbed to the column of anion exchanger and chloride ions (with minus-one-charge) are released. Ions of higher charges can be adsorbed more easily by the exchanger. Solution A form white precipitate with AgNO3, while no precipitate with BaCl2.
Step 2:
3 drops of the CuSO4 solution are passed through SP-column in Na form. A blue band is observed at the top of the column: The copper cations are adsorbed by the cation exchanger. By the addition of deionized water to the column, colorless Solution B is obtained. Solution B forms white precipitate with BaCl2. By the addition of 2M NaCl to the column, blue band at the top moves down and blue Solution C is obtained. This shows that sodium ions are adsorbed and copper ions are released because concentrated NaCl solution is used.
 Microscale Family 2013
Microscale Family 2013